Clinical Trials - 6 month
The safety and efficacy of LUNESTA (3 mg) was evaluated in a 6-month randomized, double-blind, placebo-controlled study in adults under 65 (N=828).
LUNESTA Helped Patients Fall Asleep Faster vs. Placebo as subjectively measured by patient-reported sleep latency
Primary Endpoint: LUNESTA (3 mg) was superior to placebo in decreasing patient-reported sleep latency (SL) for the month 4-6 average1,2
Patient-Reported Sleep Latency (min) for LUNESTA (3 mg) vs. placebo*
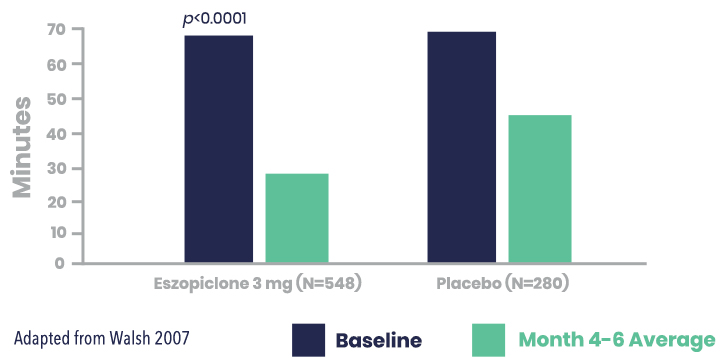
LUNESTA Reduced Wake After Sleep Onset (WASO) vs. Placebo
Secondary Endpoint: LUNESTA (3 mg) significantly improved patient-reported WASO vs. placebo for the month 4-6 average1,2
Patient-Reported WASO (min) for LUNESTA (3 mg) vs. placebo*
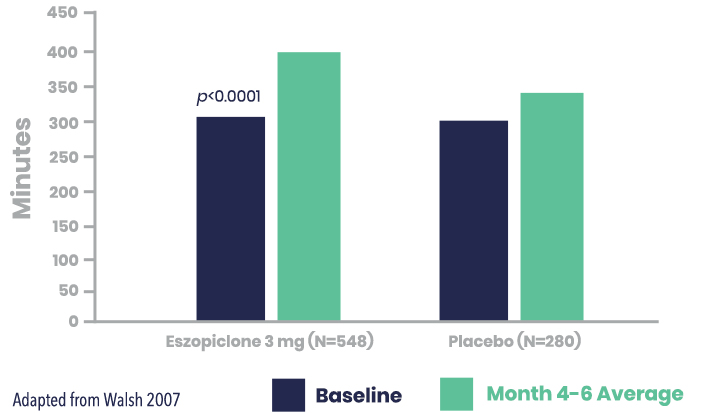
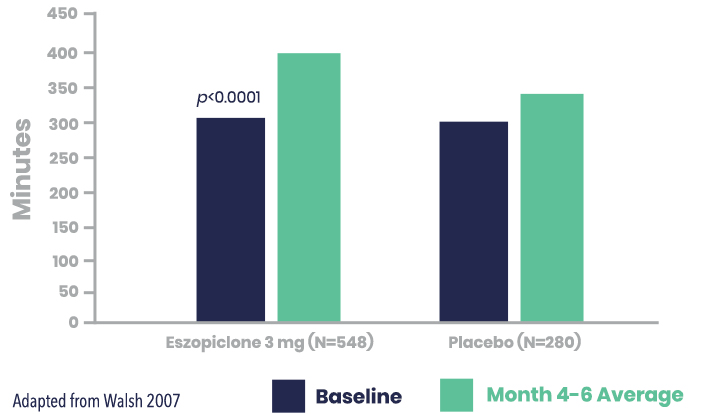
LUNESTA Increased Total Sleep Time (TST) vs. Placebo
Secondary Endpoint: LUNESTA (3 mg) significantly improved patient-reported TST vs. placebo for the month 4-6 average1,2
Patient-Reported TST (min) for LUNESTA (3 mg) vs. placebo*
The recommended starting dose is 1 mg. The dose can be increased to 2 mg or 3
mg if
clinically indicated.
Use the lowest effective dose of LUNESTA possible for the patient.