Clinical Trials - Safety Profile
LUNESTA Was Generally Well-Tolerated in Adults
Adverse Drug Reaction Overview1
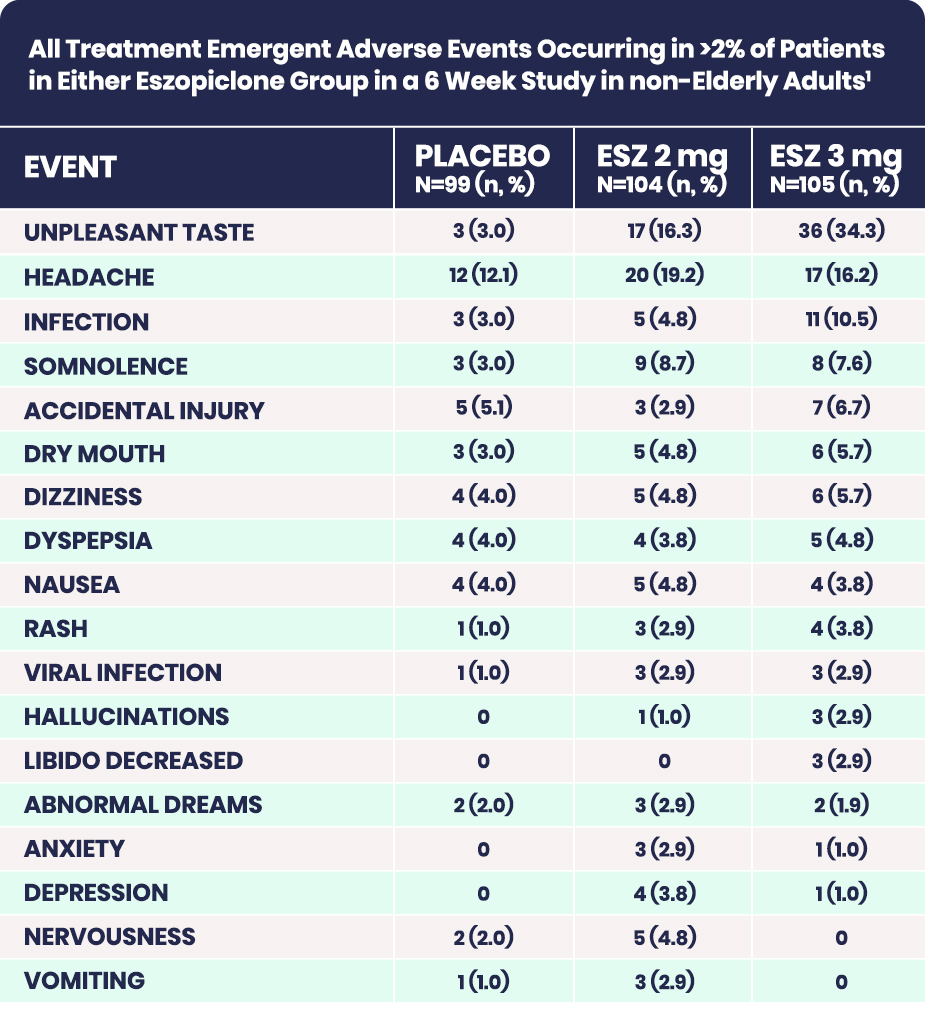
Adapted from the Product Monograph
Treatment Emergent Adverse Events in Placebo-Controlled Clinical Trials with Dosing up to 6-months in Adults under 65
The types of events reported were similar to those observed in the 6-week trial in non-elderly adults. Additional adverse events reported more frequently with eszopiclone compared to placebo included nausea (7.4% vs. 5.2%) and pharyngitis (8.1% vs. 4.4%).1
Tolerance Observations in Clinical Trials with LUNESTA
- In clinical studies with LUNESTA, no development of tolerance to any median parameter of sleep measurements was observed during treatment periods of up to 12 months1
- Development of tolerance in some patients cannot be excluded
Rebound Insomnia Effects with LUNESTA
Rebound Insomnia with LUNESTA (2 mg and 3 mg) was assessed in a 6-week adult study on the first two nights of discontinuation1,2
For both 2 mg and 3 mg, the discontinuation-emergent effect:
- was mild
- had the characteristics of the return of the symptoms of chronic insomnia
- appeared to resolve by the second night after LUNESTA discontinuation
In the LUNESTA 2 mg group, increased WASO and decreased Sleep Efficiency were observed on the first night only vs. on-treatment baseline.
LPS and WASO were significantly increased and Sleep Efficiency was decreased on the first night vs. placebo. There were no significant differences on the second night.
In the LUNESTA 3 mg group, no changes from on-treatment baseline were noted on the first night.
Sleep efficiency was significantly reduced on the first night only vs. placebo.
The recommended starting dose is 1 mg. The dose can be increased to 2 mg or 3 mg if clinically indicated. Use the lowest effective dose of LUNESTA possible for the patient.
Adverse Events in Patients with Medical Co-Morbidities
Major Depressive Disorder (MDD) or General Anxiety Disorder (GAD)1
In 2 clinical studies of non-elderly adults with MDD or GAD, spanning 8-weeks duration:
- When LUNESTA was administered in combination with a selective serotonin reuptake inhibitor, incidences of AEs were generally similar to placebo
- AEs that were reported more frequently in the eszopiclone group compared to the placebo group included:
Rheumatoid Arthritis1
In a 4-week clinical study of non-elderly adults for Rheumatoid Arthritis (RA):
- When LUNESTA was administered in adults with rheumatoid arthritis, incidences of AEs were usually similar to those reported in the 6-week clinical trial
- AEs that were reported more frequently in the eszopiclone group compared to the placebo group included asthenia (6.5% vs. 1.3%), pharyngitis (5.2% vs. 0%), and rheumatoid arthritis (18.2% vs. 9.2%)
- Treatment with eszopiclone 3 mg did not result in any worsening of the underlying RA in these subjects