Clinical Trials - 6 week
The safety and efficacy of LUNESTA (2 mg and 3 mg) was evaluated in a 6-week randomized, double-blind, placebo-controlled, parallel study in adults under 65 (N=308).
LUNESTA Helped Patients Fall Asleep Faster vs. Placebo as objectively measured by LPS
Primary Endpoint: LUNESTA (2 and 3 mg) significantly decreased latency to persistent sleep vs. placebo at 4 weeks1,2
Sleep Latency (min) for LUNESTA (2 and 3 mg) vs. placebo
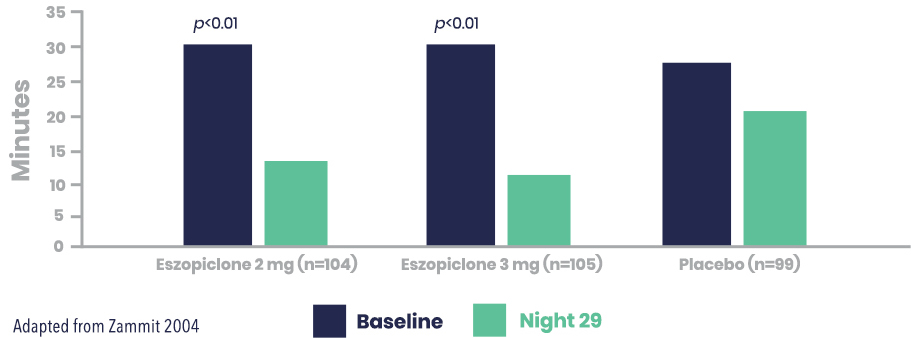
LUNESTA Improved Measures of Sleep Maintenance vs. Placebo as objectively measured by wake after sleep onset (WASO)
Secondary Endpoint: LUNESTA (3 mg) significantly reduced WASO vs. placebo1,2
Wake After Sleep Onset (min)
p<0.01
Adapted from the Product Monograph
Reduction in WASO was not statistically significant relative to placebo for eszopiclone 2 mg.
The
recommended
starting dose is 1 mg. The dose can be increased to 2 mg or 3 mg if clinically indicated. Use the lowest
effective dose of LUNESTA possible for the patient.

